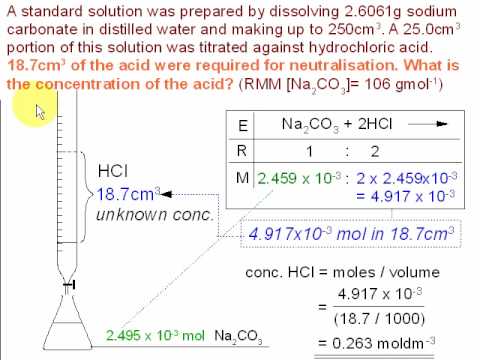
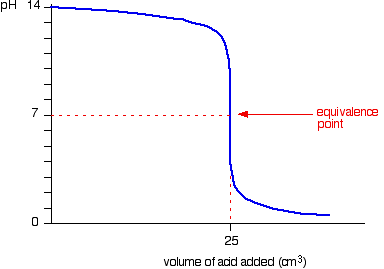
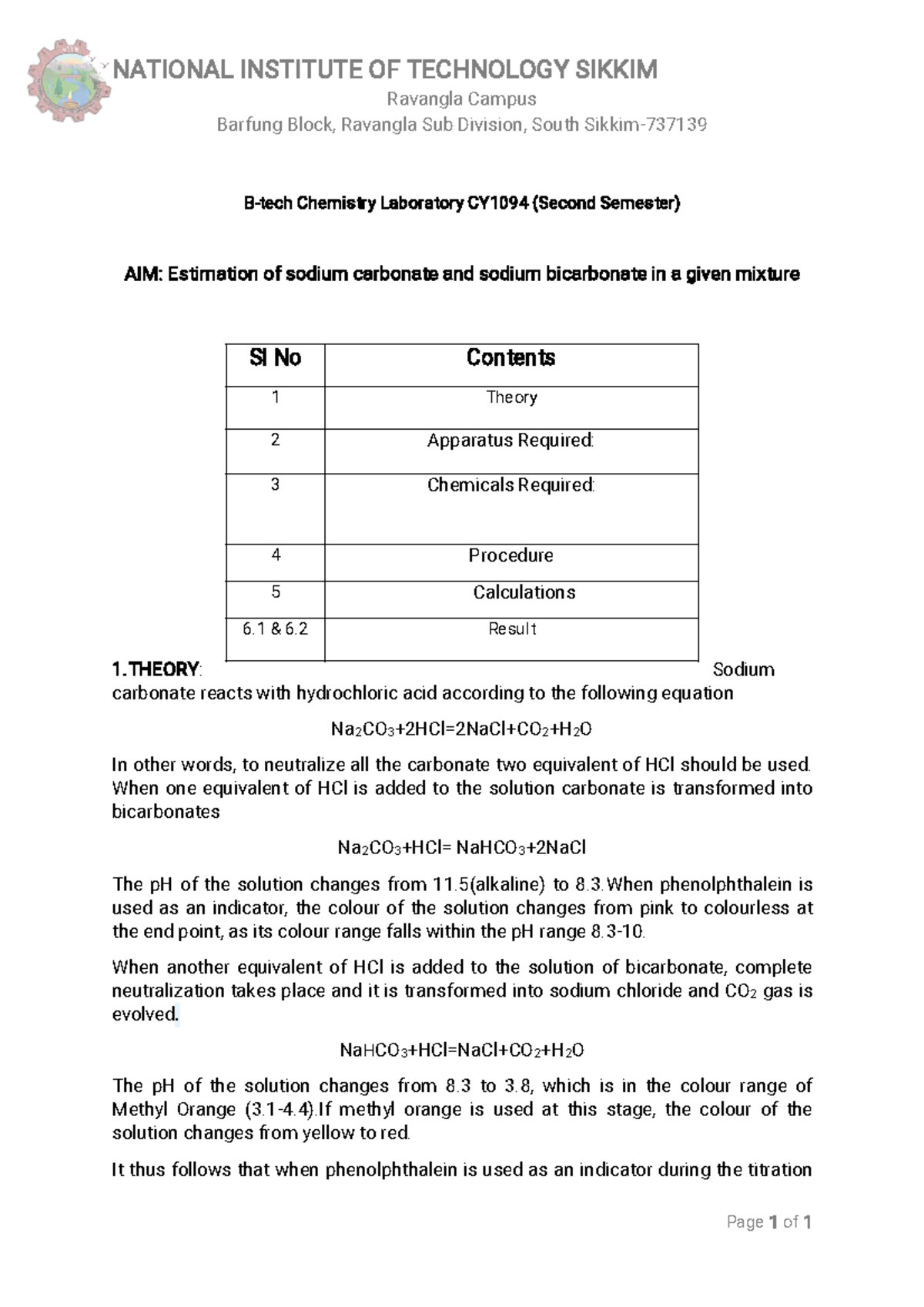
SOLVED: Calculate alkalinity (mmol/L) of water to which sodium carbonate (Na2CO3) was added so that its concentration is 1.9 g/L and pH = 8.5. Dominant form of carbonate at this pH= HCO-3

Determination of sodium carbonate and sodium bicarbonate in a solution containing both salts | BPC2113 - General Chemistry - IMU | Thinkswap

400 ML Solution Of Carbonate (density, Contains 22g Of Sodium Calculate The Mass Per Cent Of Sodium Carbonate In | Sodium Percarbonate 400 Gr | tk.gov.ba
![Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ] Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ]](https://haygot.s3.amazonaws.com/questions/1762025_1744330_ans_4bbe4fbaf6204e3b9a3b783eee4440f1.jpg)
Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ]
![SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen](https://cdn.numerade.com/previews/ef6e1b45-03cf-464d-9e55-544d05108206_large.jpg)
SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen

physical chemistry - Which formula can be used to calculate the exact hydronium concentration present in sodium hydrogen carbonate solution? - Chemistry Stack Exchange

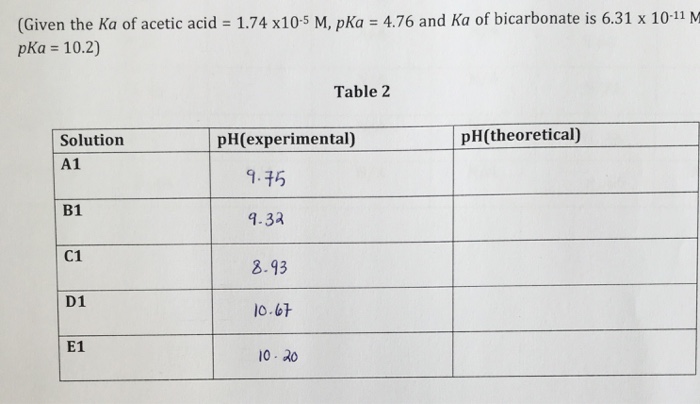

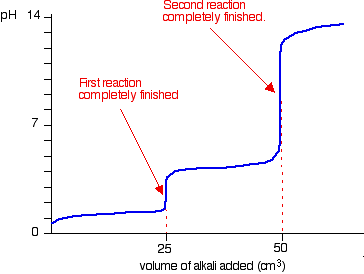
![PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/aaa98c1f9de4c398b6a057bf4936ea76736034c7/1-Table1-1.png)