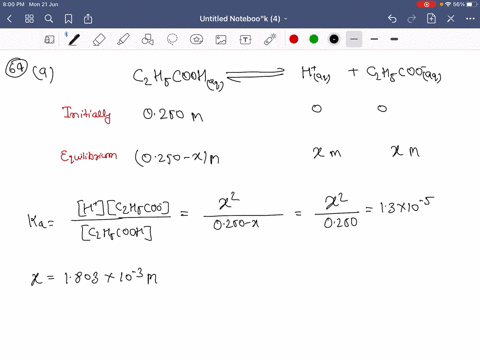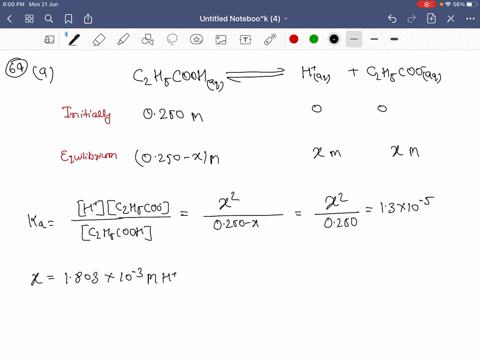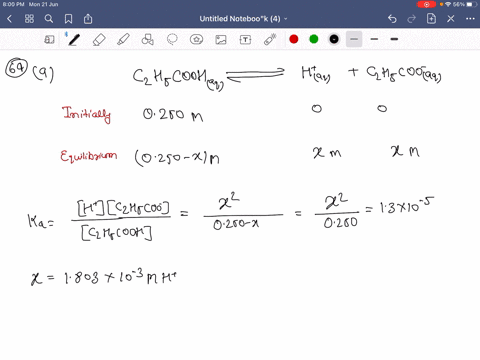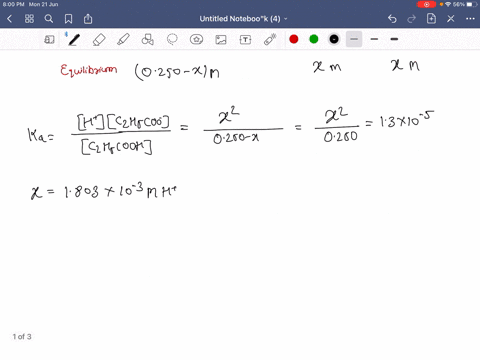
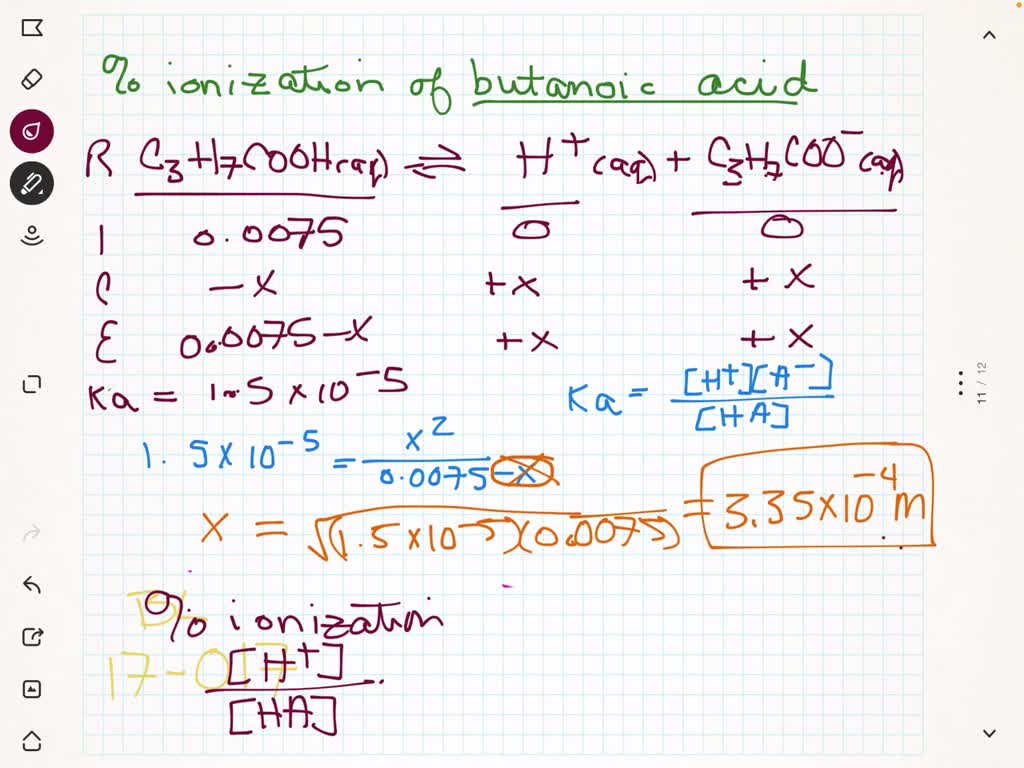
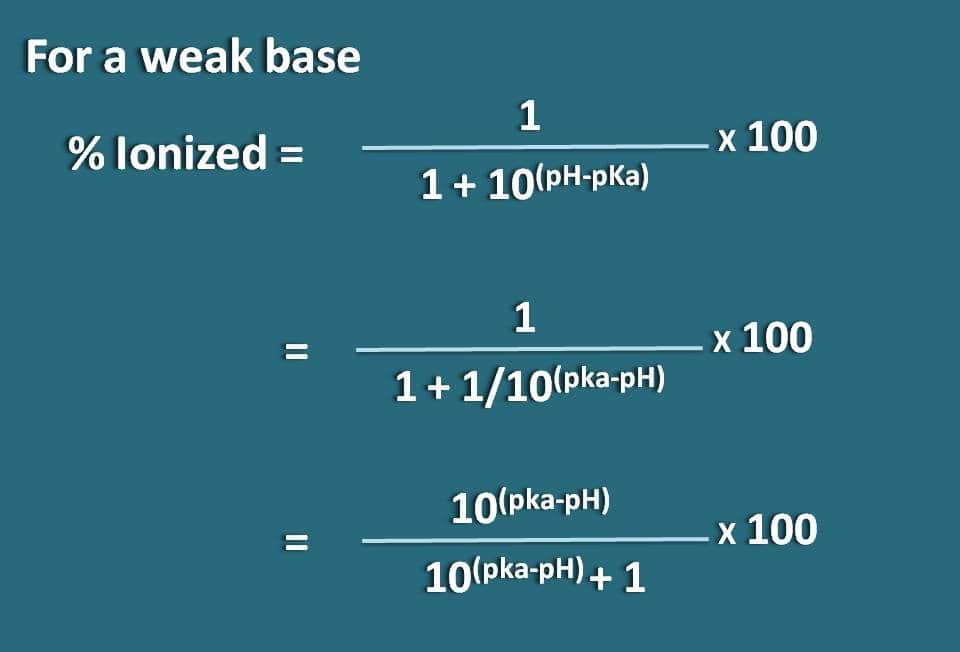
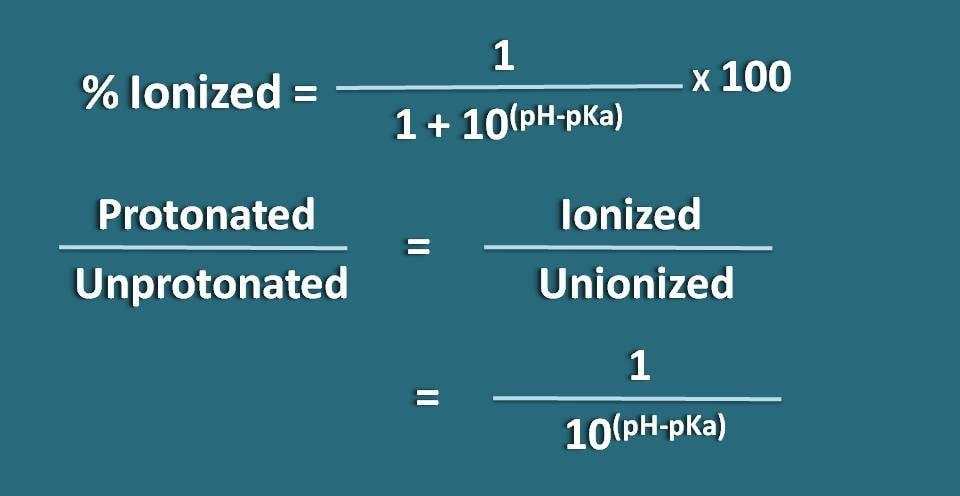
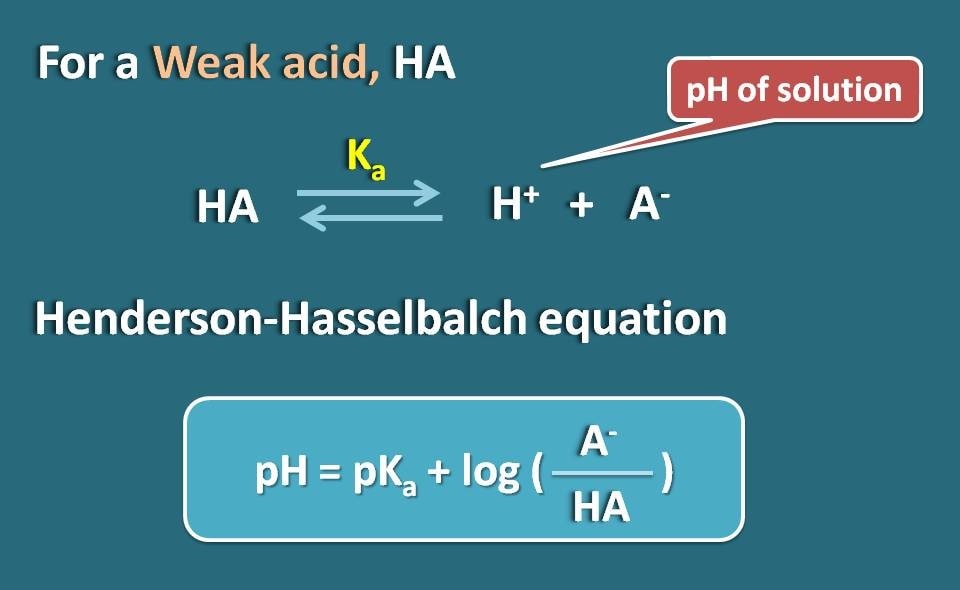
SOLVED:(a) Calculate the percent ionization of 0.125 M lactic acid (Ka=1.4 ×10^-4). (b) Calculate the percent ionization of 0.125 M lactic acid in a solution containing 0.0075 M sodium lactate.
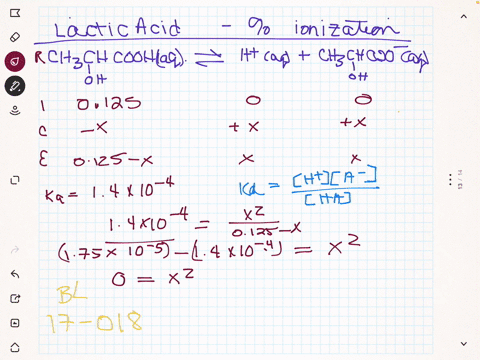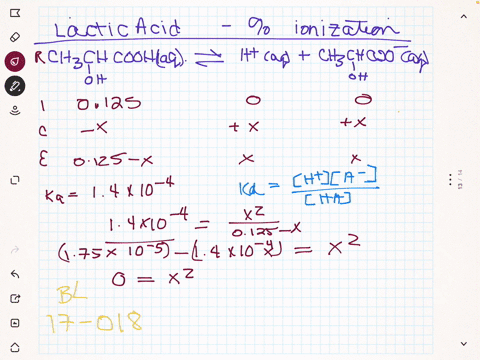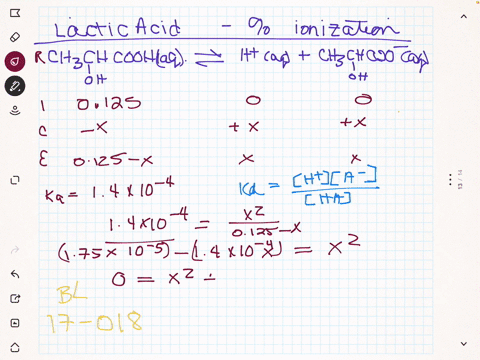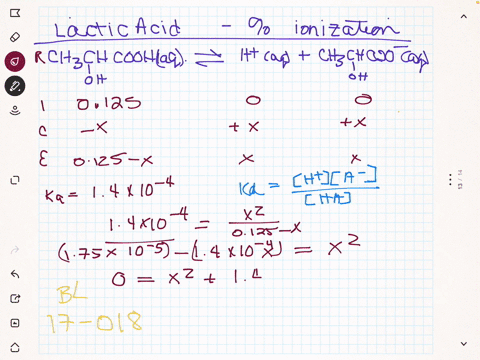
SOLVED:(a) Calculate the percent ionization of 0.125 M lactic acid (Ka=1.4 ×10^-4). (b) Calculate the percent ionization of 0.125 M lactic acid in a solution containing 0.0075 M sodium lactate.
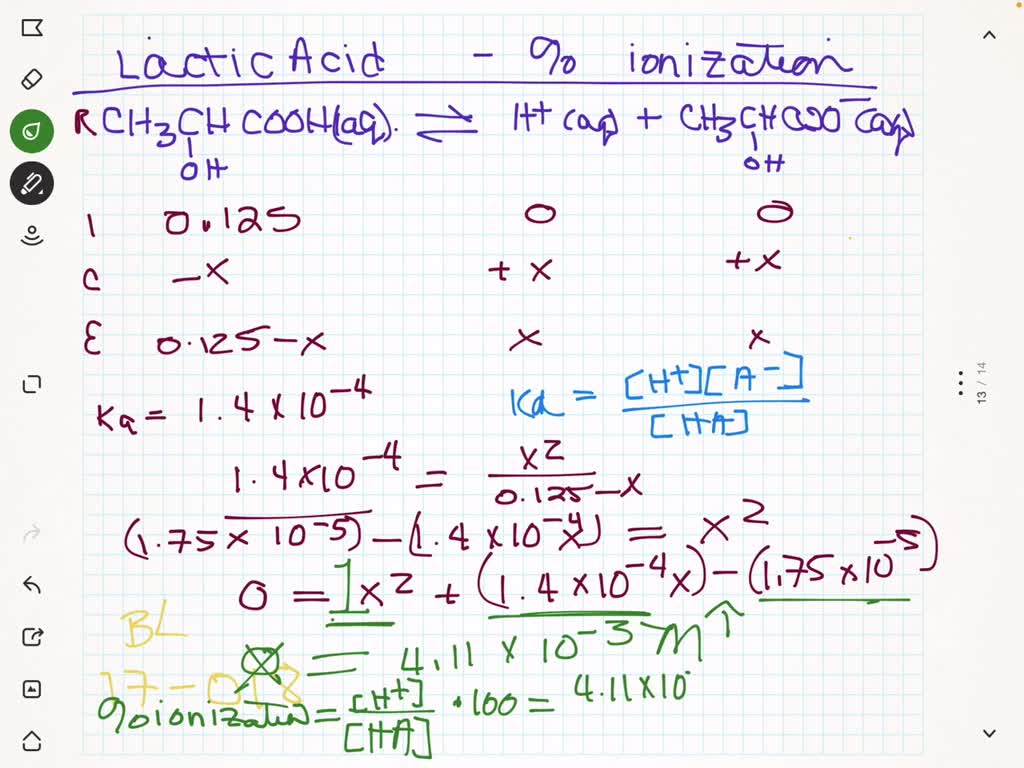
SOLVED:(a) Calculate the percent ionization of 0.125 M lactic acid (Ka=1.4 ×10^-4). (b) Calculate the percent ionization of 0.125 M lactic acid in a solution containing 0.0075 M sodium lactate.
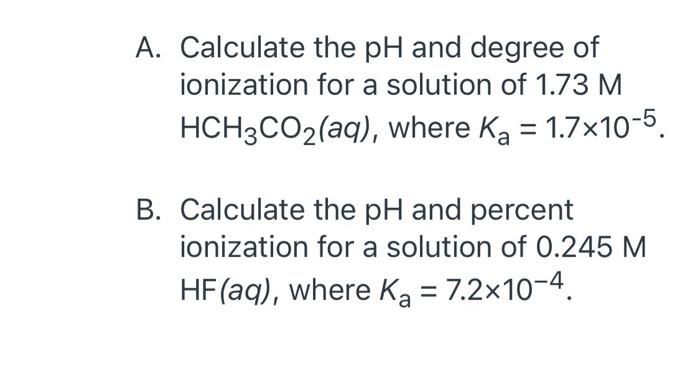


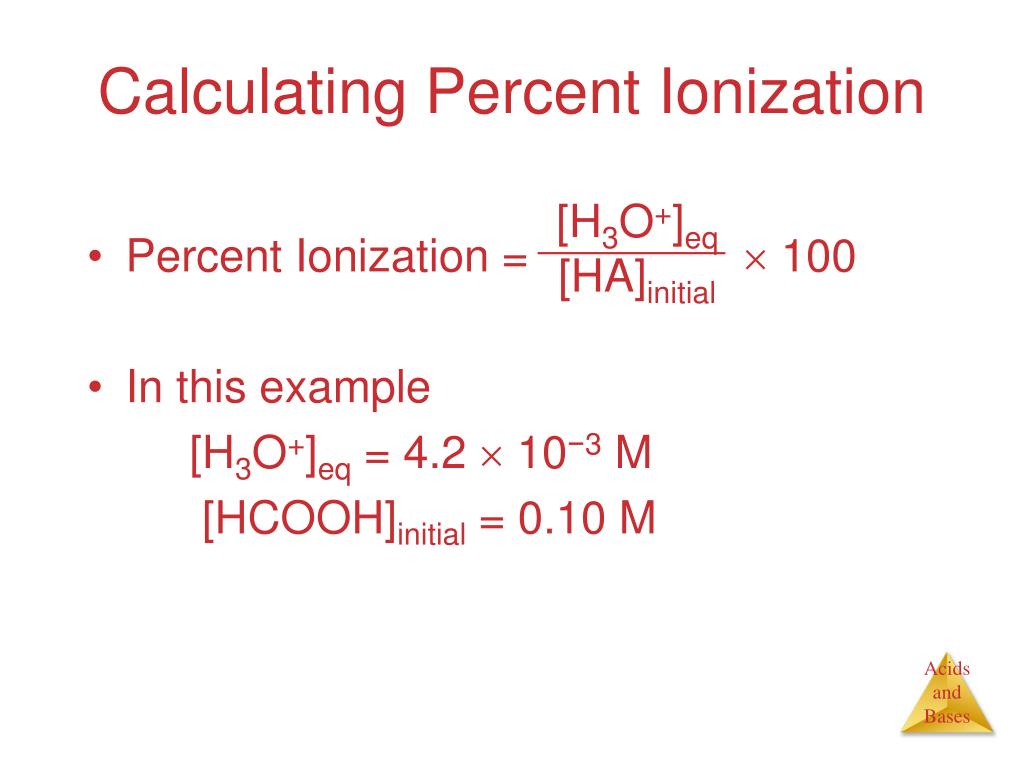



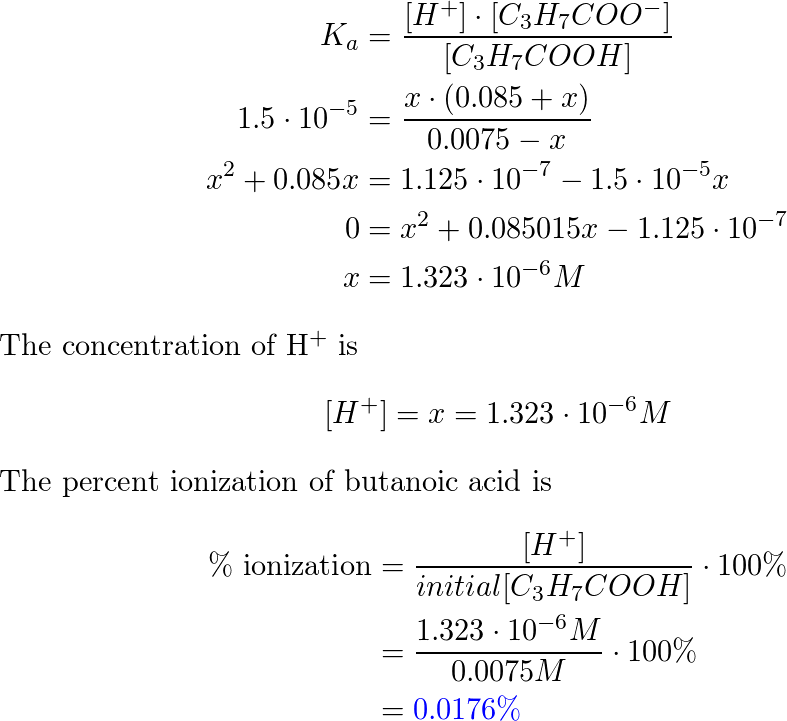


![Solved Percent ionization = [H3O+]equil/[HA}init Times 100% | Chegg.com Solved Percent ionization = [H3O+]equil/[HA}init Times 100% | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F111%2F1110fa34-0985-4a45-9e25-717b01b554f8%2Fimage)




