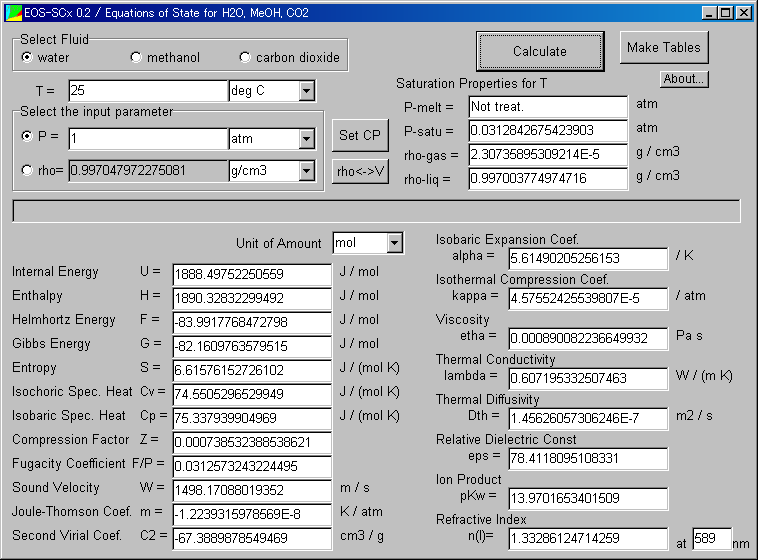
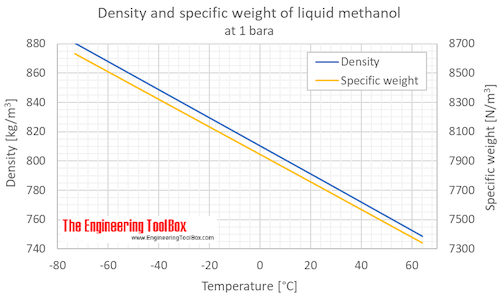
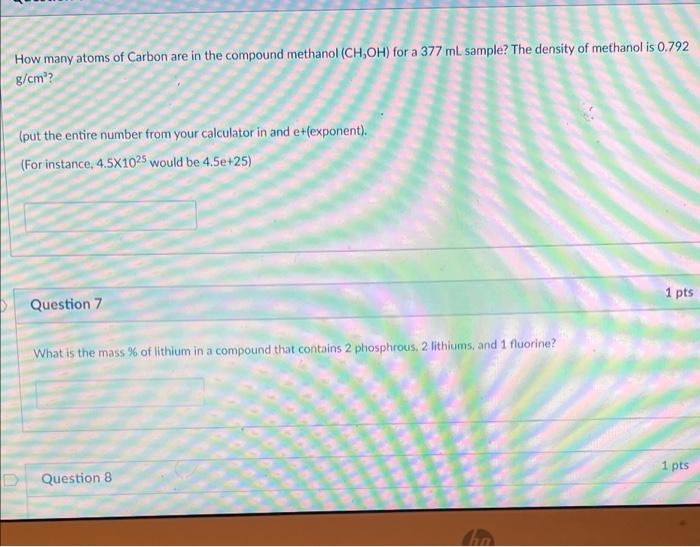
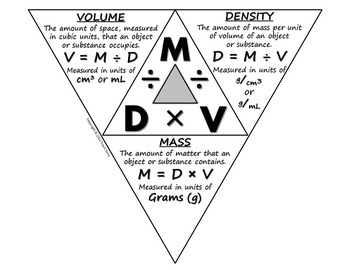
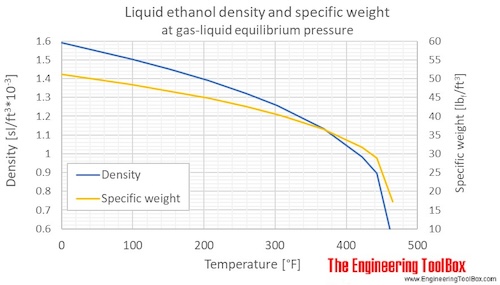
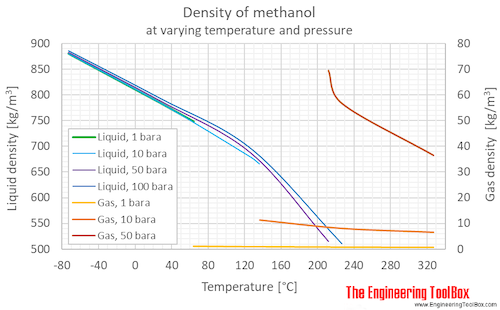
Calculate the number of moles of methanol in 5 litres of its 2 m solution if the density of solution is 0.981 kg (Molar mass of methanol=32.0g mol^(-1).

Determining weight-percent methanol in water from specific gravity and temperature - Chemistry Stack Exchange

SOLVED: Calculate the molality of methyl alcohol (CH3OH) in a solution prepared by dissolving 21.8 g of methyl alcohol in 900 mL of water (density of water = 1.00 g/mL). Select all

SOLVED:The density of acetonitrile (CH3 CN) is 0.786 g / mL and the density of methanol (CH3 OH) is 0.791 g / mL . A solution is made by dissolving 22.5 mL

Calculate the moles of methanol in 5 litres of its 2 m solution, if the density of the solution is 0.981 | Solutions, Mole, Density

Determining weight-percent methanol in water from specific gravity and temperature - Chemistry Stack Exchange

Calculate the number of moles of methanol in 5 litres of its 2 m solution if the density of solution is 0.981 kg (Molar mass of methanol=32.0g mol^(-1).