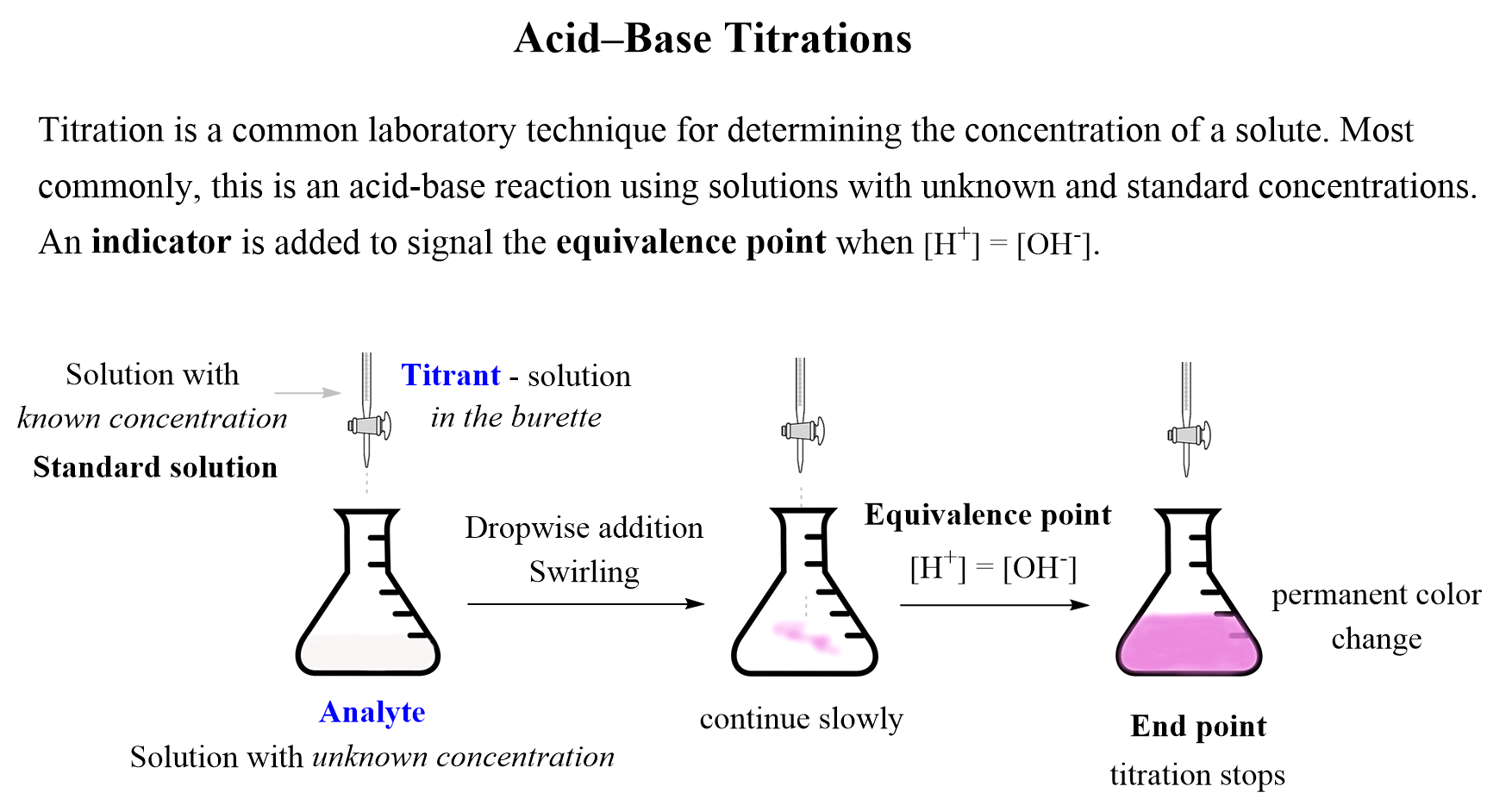
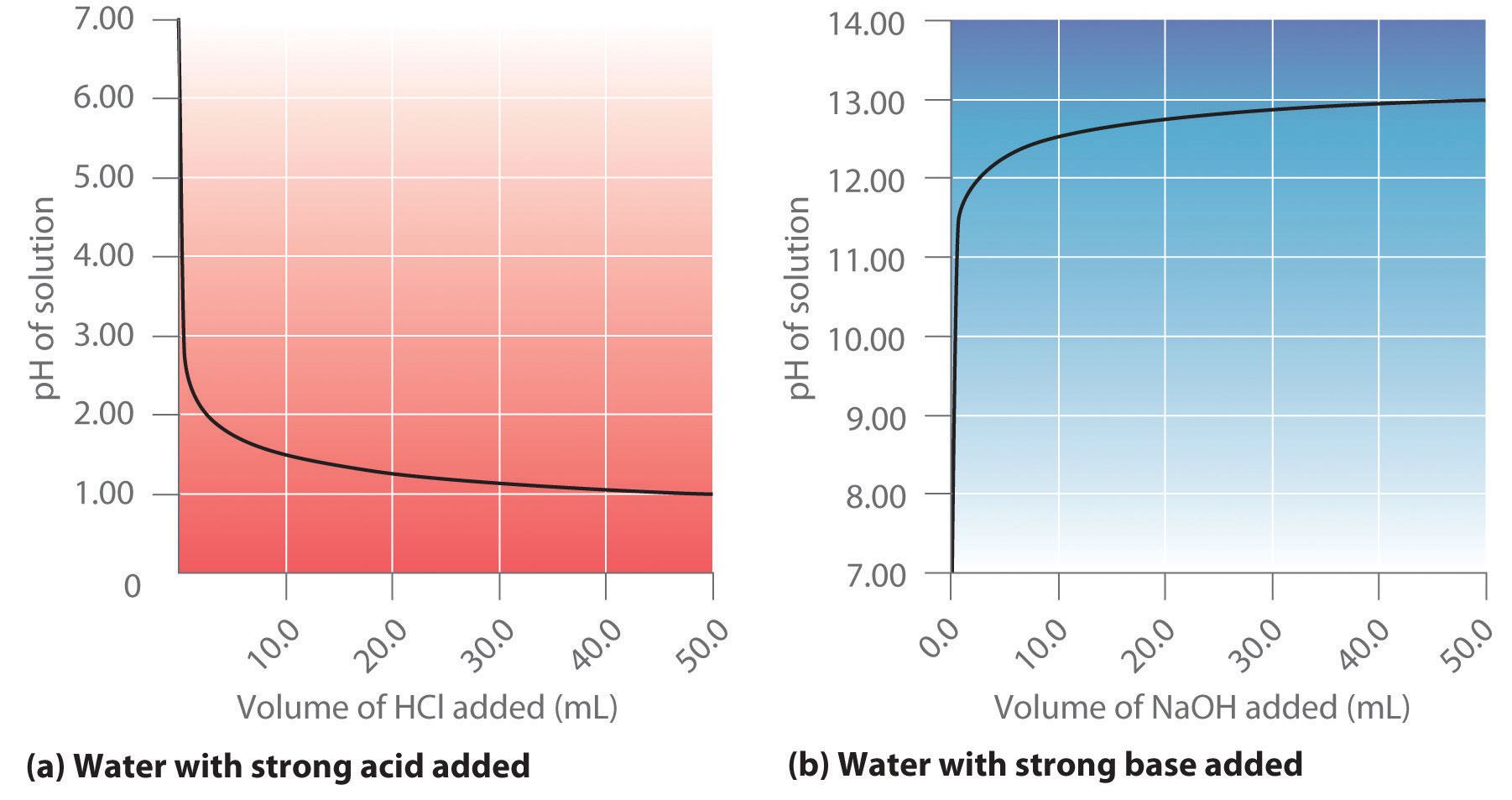
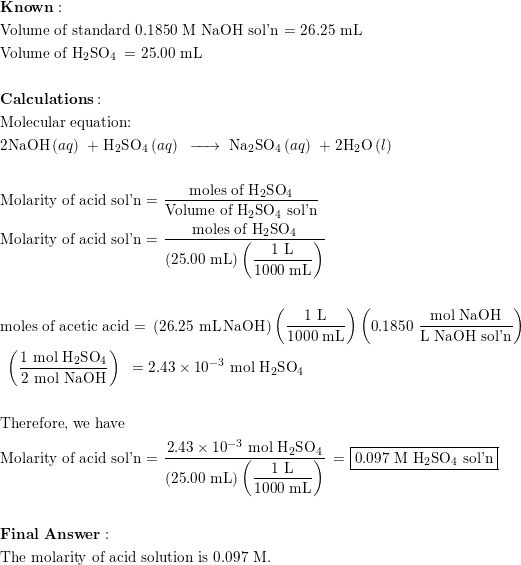
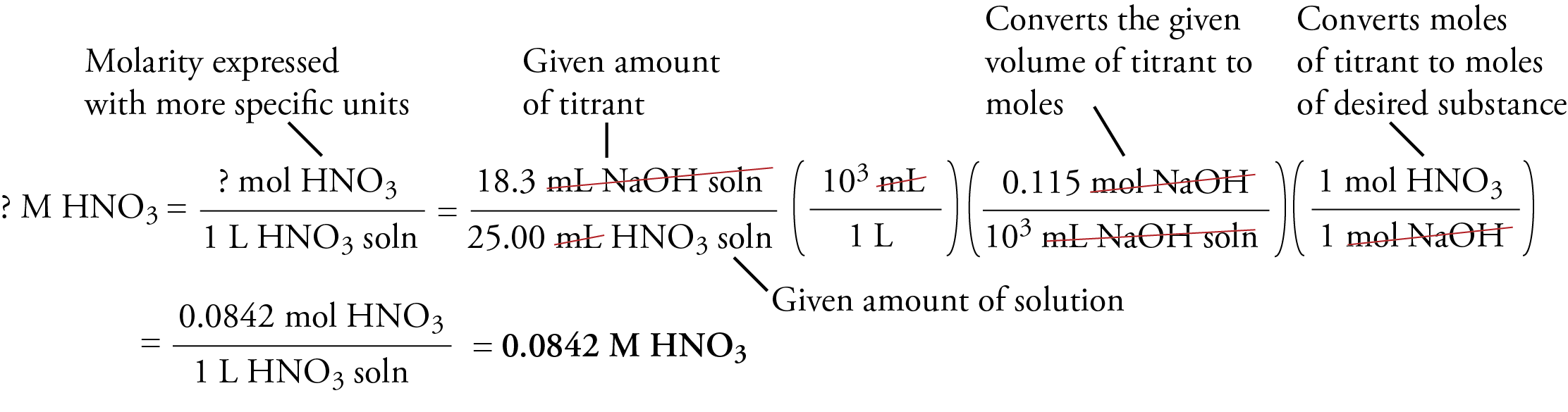
Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.
What volume of 0.1mol/dm3 hydrochloric acid will be required to neutralize 20cm3 of 2.0mol/DM3 sodium hydroxide? - Quora
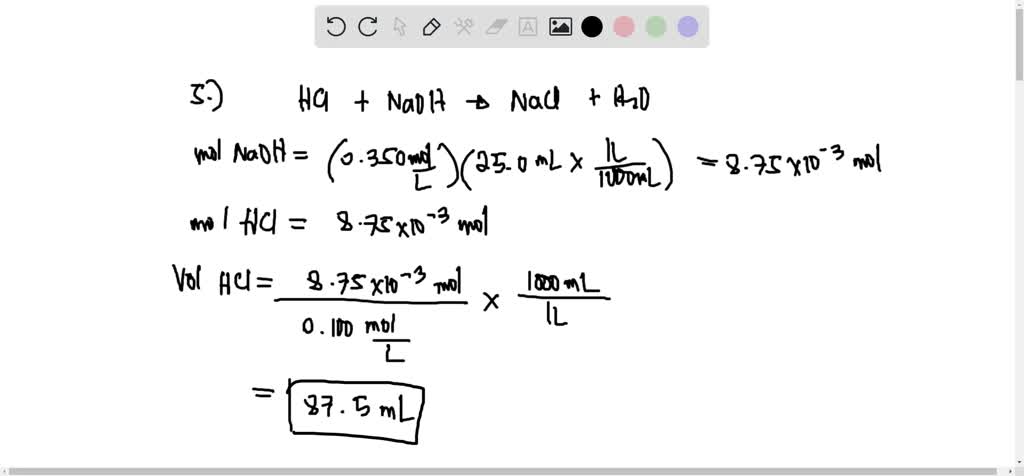
SOLVED: 5. Calculate the volume of 0.100 M HCI solution needed to neutralize 25.0 mL of 0.350 M NaOH solution. (Answer: 87.5 mL) Calculate the volume of 0.100 M HzSO4 solution needed
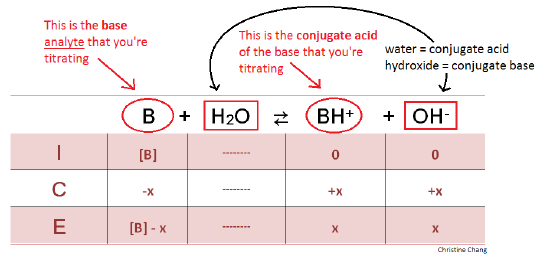
![Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download](https://images.slideplayer.com/39/10846268/slides/slide_7.jpg)
Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download
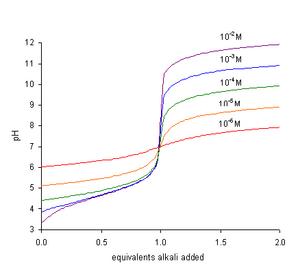
![Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download](https://images.slideplayer.com/39/10846268/slides/slide_2.jpg)
Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download

Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa

Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.
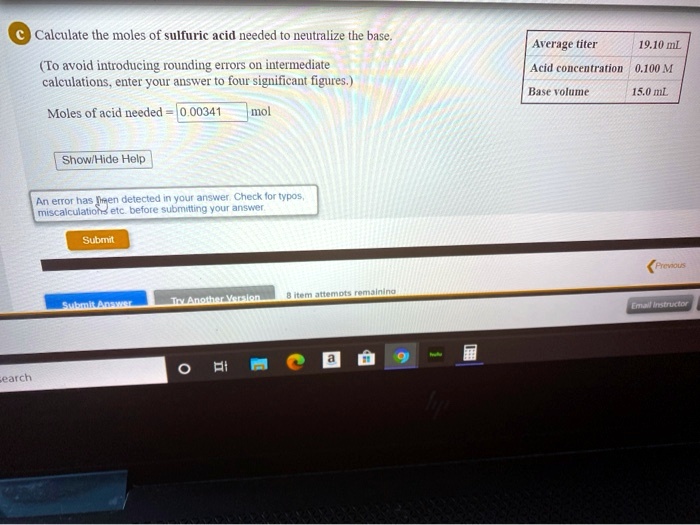
SOLVED: Calculate tlie moles of sulfuric acid needed t0 neutralize the base. Avcrage titer 19.10 mL (To avoid introducing rounding errors On intermediate calculations enter YOUr answer t0 four significant figures, )
![Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download](https://slideplayer.com/10846268/39/images/slide_1.jpg)